Tuesday, 16 July 2019|Source:CHINA CHAMBER OF COMMERCEFOR IMPORT&EXPORT OF MEDICINES &HEALTH PRODUCTS|Author:By LI Hui, Department of Information
Generic drug approvals obtained by Chinese companies in the U.S. are on the rise. From 2006 to 2010 and from 2011 to 2014, Chinese companies obtained a total of 6 and 25 ANDA approvals respectively. After 2015, the newly approved ANDAs amounted to at least 10 to 20 every year (ANDA batch number of different specifications under the same variety was calculated as one ANDA), enjoying a rapid growth trend. By the end of 2018, Chinese companies had 455 valid approval numbers (excluding tentative approvals), covering nearly 200 products from different companies.
In 2018, the ANDA approvals of Chinese companies reached a record high of 71, 16 of which are provisional approvals. Among them, HEC, Huahai Pharmaceutical and Humanwell Healthcare are the top three companies with 12, 11 and 6 ANDA approvals respectively (ANDA batch number of different specifications under the same variety was calculated as one ANDA). Besides the climbing number of ANDA approvals, a multiple of Chinese companies obtained ANDA approval for the first time in 2018, such as Shijiazhuang Yiling Pharmaceutical Co., LTD., Shandong New Time Pharmaceutical Co., LTD., Shuangcheng Pharma, Boya Seehot, Shanghai Desano Pharmaceuticals Co., Ltd. (Desano), and Qilu Tianhe Pharmaceutical
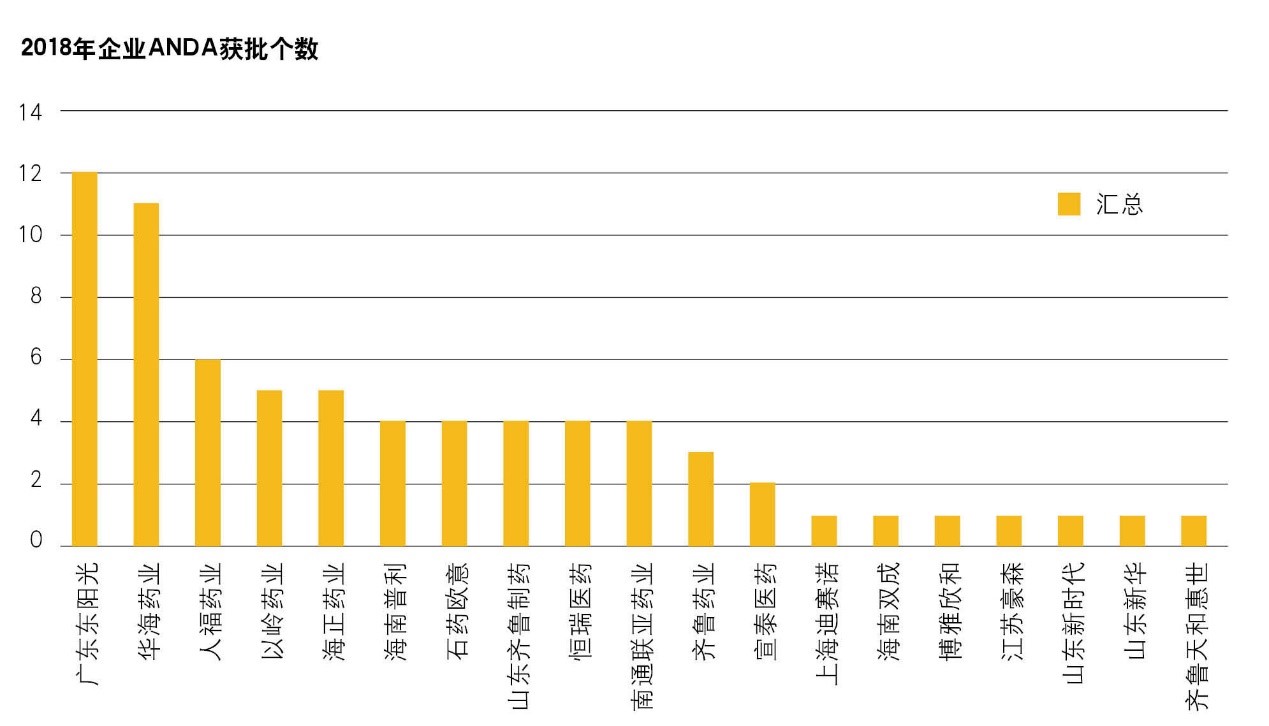
Chart: Numbers of ANDA Approvals of Chinese Pharmaceutical Companies in 2018
By LI Hui, Department of Information
Source:CCCMHPIE